Oxygenates
Oxygenates refer to oxygen containing compounds, which are used as fuel components. Traditionally oxygenates refer to gasoline components, such as ethanol, fuel ethers, and recently also butanol. Gasoline oxygenates are described in separate Chapters, and here only diesel oxygenates are discussed.
Oxygenates for gasoline
Oxygenates for diesel
Oxygenates are considered as diesel fuel components mainly due to their capability to reduce particulate matter emission (PM). When based on renewable feedstocks, oxygenates can also tackle with the challenges of climate change and energy security.
A number of different oxygenates, for example alcohols, ethers, esters, and carbonates, have been explored as diesel fuel components (Table 1). Many studies have covered a comprehensive set of oxygenates, e.g. Pecci et al. (1991) reviewed some 80 oxygenates, Natarajan et al. (2001) 71 oxygenates and Delfort et al. (2002) 18 oxygenates. In addition, numerous studies have focused on specific oxygenate groups (Nylund et al. 2005).
Table 1. The general structure of oxygenates
| R-OH | Alcohols |
| R-O-R | Ethers |
| R-O-R-O-R | Glycol ethers |
| R-O-C-O-R | Acetals |
| R-C(=O)-O-R | Esters |
| R-O-C(=O)-O-R | Carbonates |
| R hydrocarbon chain; C carbon; O oxygen | |
Many fuel properties of oxygenates need consideration when evaluated as diesel fuel components, for example cetane number, flammability, volatility, density, viscosity and lubricity. Oxygenates are generally polar in nature, which may lead to compatibility problems when blended with diesel fuel. The most common diesel oxygenates are fatty acid esters. Low molecular weight oxygenates are typically not compatible with diesel fuel, whereas some heavier alcohols, ethers, and acetals are considered as promising diesel blending components. Special engines might be capable to use "non-compatible" oxygenates, however, an optimum oxygenate would be compatible with existing engines and infrastructure.There are a number of fuel properties, both physical and chemical, which are important for the proper operation of a diesel engine. Regulations and standards control diesel fuel quality, and these requirements should be met also by when oxygenates are blended in diesel fuel.
Fuel properties of oxygenates depend on, e.g., length and type of alkyl chains. Oxygenates with higher molecular weight often have higher density, higher boiling point, higher viscosity, better lubricity, lower volatility, and lower flammability than respective oxygenates with lower molecular weight. Therefore, oxygenates with higher molecular weight are preferred as diesel fuel components.
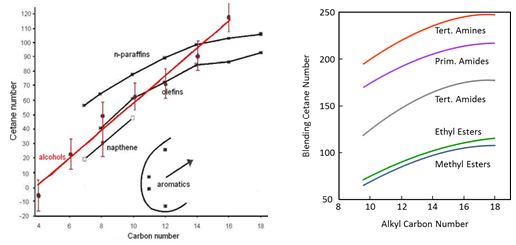
The diesel process is based on auto-ignition of the fuel, which is described by cetane number. In general, poor ignition quality leads to improper combustion and increased exhaust emissions. Ignition improver additives may increase cetane number to some extent. A minimum cetane number of 51 is defined for diesel fuel in Europe. As concerns hydrocarbons, normal alkanes, called straight-chain paraffins, have the highest cetane numbers, whereas aromatics have the lowest cetane numbers. Ignition quality improves as the chain length increases (Figure 1). This applies also to oxygenates with same functional groups and similar branching of alkyl chain. Blending cetane number is reported to increase with increasing alkyl chain up to di-n-hexyl ether. Branching of the alkyl chain depresses cetane number of oxygenates. High cetane numbers have been observed for molecules with three ether groups in structure, and for molecules with oxygen atom at central or at omega positions. Cetane numbers of esters are quite modest. (Pecci et al. 1991, Stournas et al. 1993).
Figure 1. The effect of molecular size on cetane number (Shell Lexikon, Stournas et al. 1993).
Distillation range of diesel fuel is around 180 - 340 °C and new components are desired to fall into this range to ensure proper combustion in diesel engine. However, traditional distillation methods may not be applicable for oxygenates (Smith et al. 2008). Storage and handling regulations for fuels are based on the flash point, which is the limit temperature for the formation of an ignitable air-fuel vapor mixture. In Europe, flash point of diesel fuel is higher than 55 °C.
Hydrocarbons, especially alkanes, are non-polar compounds, whereas oxygenates are polar in nature. The most polar oxygenates, e.g. methanol and ethanol, are not miscible with diesel fuel with exception of emulsions. Less polar oxygenates, for instance n-pentyl ether, are miscible with diesel fuel. Miscibility depends on the chemical structure, oxygen content, and on the physical properties of substances (“like dissolves like”). Increasing aromatics content of diesel fuel improves the solubility of oxygenates with diesel fuel. (McCormic 2001). Water affinity of oxygenates is higher than that of hydrocarbons. In addition, oxygenates tend to be hygroscopic absorbing water from ambient air. Dissolved water is not harmful for an diesel engine, whereas phase separation presents a considerable risk. Also corrosion of materials may occur with oxygenates. For many oxygenates lubricity is a problem. This can be alleviated by using additives. Some oxygenates may form aggressive compounds, such as acids.
Diesel fuel injection system is based on volumetric principle. Therefore lower density fuel typically leads to reduced maximum power output and higher volumetric fuel consumption. For oxygenates, energy content is typically lower than that for hydrocarbon fuels. Low viscosity of fuel may cause fuel leakages, whereas high viscosity of fuel may overload the injection system. Typically, oxygenates do not contain aromatics or sulfur, which is a benefit as regards exhaust emissions and exhaust aftertreatment devices. Cold properties of oxygenates improve with increasing branching, and worsen with increasing number of oxygen atoms. (Pecci et al. 1991).
Exhaust emissions with diesel oxygenates
For diesel engine, NOx and PM emissions are bottlenecks. These can be reduced by using emission control technologies or by using tailored fuels, such as oxygenates. PM reductions of 3 - 15%, and even 67%, have been observed at 1% oxygen level in diesel fuel. Soot-free combustion may be achieved at sufficient oxygen to carbon ratio, for example at 30 - 40 wt-% oxygen content. NOx emissions tend to increase slightly with oxygenates, depending on molecule and on engine characteristics. Carbonyl emissions may increase with oxygenates, but not necessarily if high cetane number is combined with over-lean mixture zone and high combustion temperature. In the automotive engines, the dominating process for NOx formation is oxidation of nitrogen in air at high temperatures (Zeldovich mechanism). Particles are formed in the rich zones in the fuel jet via pyrolysis of the fuel. (Boot et al. 2009, Gonzales 2001, Serdari 2000, Hess 2000, Chang 1997, Bertoli et al. 1997, Karas 1994, Hashimoto et al. 1991).
A number of mechanisms have been presented to explain reduction of PM emission with oxygenates. Oxygenated fuels bring oxygen in the combustion process. Many studies emphasize the differences between the functional groups of oxygenates. The role of oxygen entrained from ambient has also an impact, as well as the engine characteritics, load mode and test conditions. In older engines with fully mechanical injection systems, increase in fuel density and viscosity advanced injection timing leading to higher NOx emissions. In new electronically controlled injection systems fuel properties do not influence the start of injection. The load-dependent ability of oxygenates to reduce PM emissions might be related to changes in air-to-fuel ratio and combustion temperature. Flame temperatures of aromatic hydrocarbons are higher than those of paraffins and alcohols. Evaporation of alcohols cools the air-fuel mixture. Temperatures may be locally reduced due to higher evaporation heat of in the reaction zone where soot appears. Oxidation molecules may be available earlier in rich and high temperature zones enabling soot oxidation already in the phases of soot formation. High cetane number of fuel generally leads to decreased PM emissions. In some cases cetane number has been determining factor at lower loads, whereas oxygen content has been dominating at higher loads. The high cetane number and oxygenates could be beneficial at high EGR ratios. For some engines, oxygenates with low cetane number have led to low PM emissions. (Nabi and Hustad 2010, Janssen et al. 2009, Boot et al. 2007, 2009, Delfort et al. 2002, Bertoli et al. 1997, Giavazzi et al. 1991).
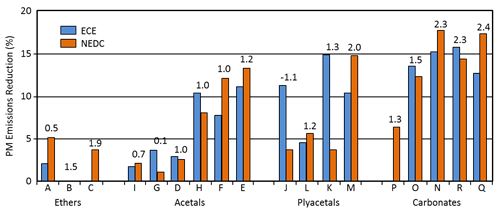
Delfort et al. (2002) concluded that the PM emission depends primarily on the functional group of oxygenate. Within each functional group, oxygen content is a key parameter based on a study with light-duty direct-injection car (Figure 2). The best performance was observed for fuels having the highest oxygen contents, but also some low-oxygen fuels reduced PM emission efficiently. (Boot et al. 2007, Bowman 2006, Delfort et al. 2002, Karas 1994).
Figure 2. The effect of oxygen is important within each chemical group of oxygenates (Delfort et al. 2002)
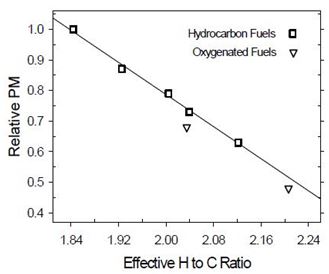
PM emission depends on hydrogen to carbon ratio for hydrocarbons fuels, whereas for oxygenated fuels PM emission is lower than could be explained by only carbon to hydrogen ratios of fuels (Figure 3). Strong carbon-oxygen bonds are assumed to prevent carbon from efficient soot formation. In principle, 30% of oxygen in esters is unavailable for eliminating soot precursors (one C atom per two O atoms), whereas C-O bonds protect one C atom per O atom. However, contradictory results have been obtained for these molecules. This could be explained if role of the C-O-C bonds was higher at premixed combustion, whereas role of the O-C=O bonds would be dominating at mixing-controlled (diffusion) combustion. Other structural parameters of molecules may also have an impact. Soot reduction efficiency is claimed to be highest when oxygen is in the middle of molecule, carbon chain is short (lower boiling point), no branches exist and when ethylene and methyl radicals are produced instead of larger alkenes. For high-molular weight esters, PM emission seems not to be dependent on branching. With oxygenated fuels, less soot may be achieved without significant differences in combustion phasing or heat release. A combination of oxygenated diesel fuel and emission control technologies may result in very low exhaust emissions. In general, non-aromatic fuels with simple chemical structure and short carbon-carbon chain burn cleanly. (McEnally and Pfefferle 2011, Boot et al. 2009, Xu et al. 2006, Mueller et al. 2003, McCormic 2002, Delfort et al. 2002, Natarjan 2001, Gonzales 2001, Yeh et al. 2001, Bertoli et al. 1997).
Figure 3. Relationship between hydrogen to carbon ratio of fuel and PM emission (Natarajan 2001)
It is difficult to rate best diesel oxygenates based on miscellaneous, partly contradictory results. However, many promising oxygenates have been found, such as alcohols (C9-C12), ethers (DNPE), maleates (DBM), glycol ethers (TPGME) and carbonates. Particularly low PM emissions have been achieved for oxygenates blended with paraffinic fuel (Frijters et al. 2006). In this Chapter, selected examples of oxygenates considered as diesel components are referred.
Esters and derivatives
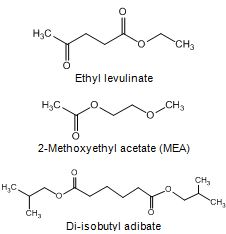
One of the esters considered as diesel fuel components is ethyl levulinate, which can be produced from cellulosic materials. However, cetane number of neat ethyl levulinate is poor, below 10. (McCormic 2002, Biofine Inc.).
2-Methoxyethyl acetate (MEA) at 20% blend in diesel fuel reduced smoke, CO and HC emissions, but had only slight effect on NOx emission in the tests with a single-cylinder engine. The ignition delay and duration of combustion were shorter for MEA than for diesel fuel. (Yanfeng et al. 2007). Hilden et al. (2001) reported that di-isobutyl adibate resulted in lower exhaust emissions than di-butyl phthalate. Lin and Huang (2003) reported that NOx, CO, and CO2 emissions decreased, but brake-specific fuel consumption increased with the ethylene glycol mono-acetate as a fuel component for marine diesel engines.
Blending cetane numbers of fatty acid derived amides (R-C(=O)NR´R´´) and amines (R-NH2) are high (Figure 1). For tertiary fatty amides at 5% concentration in diesel fuel, PM emissions reduced in the tests with a Petter engine. (Stournas 1993).
Glycerol is formed as a side-product in FAME production. Glycerol derivatives, such as glycerol-tert-butyl ethers (GTBE) have been studied as diesel fuel components. With di-butoxy glycerol, PM emissions decreased, but NOx emissions increased. (Di Serio et al. 2010, García et al. 2008, Boot et al. 2007, Spooner-Wyman et al. 2003, Karas et al. 1994).
Alcohols
Butanol isomers are lower-boiling compounds (82 - 118 °C) than diesel fuel, and primarily considered as gasoline blending components. With 10 vol-% butanol in diesel fuel, cetane number and lubricity fall below requirements. When fuel contains also 10 vol-% FAME, cetane number, viscosity and lubricity may be sufficient, but flash point remains low (44 °C). 20% and 40% blends of n-butanol showed low smoke and NOx emissions in a common rail, direct-injection EGR equipped diesel engine (Valentino et al. 2012, Wadumesthrige et al. 2010, Zöldy et al. 2010).
A blend containing 5% of rapeseed methyl ester and 10% of heavy alcohols, called Agrodiesel 15, is a stable solution meeting diesel standards. Lower PM and PAH emissions have been achieved with Agrodiesel 15 than with Swedish Environmental Class 1 diesel fuel. A long-term test and a field test of one year with buses showed no problems. (Petterson 2005, Golubkov 2005).
Yeh et al. (2001) studied various esters, carbonates, alcohols, and ethers at 2 wt-% oxygen level, and found isodecanol as the efficient oxygenate in reducing PM emission. Also a paraffinic component (IsoparTM) reduced PM emission to some extent, but not as effectively as isodecanol. Janssen et al. (2009) reported that in advanced combustion systems 1-decanol could reduce soot emissions up to 90% depending on load point. All oxygen containing compounds do not effectively reduce PM emissions, for example, aromatic 1-phenyl ethanol and 1-cyclohexyl ethanol (Karas 1994).
Ethers, acetals, ketones
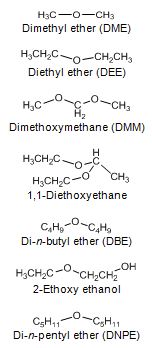
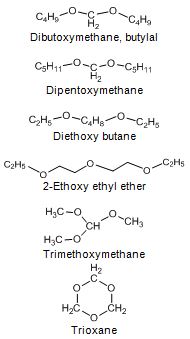
Diethyl ether (DEE) and di-methoxymethane (DMM) have been studied as diesel fuel extenders. Boiling points of these ethers are very low, 35 °C for and 42 °C. Acetal (1,1-dietoxyethane, derived from ethanol) is a light compound with a boiling point of only 103 °C and a flash point of -20 °C. Di-n-butyl ether (DBE) has a boiling point of 141 °C, which is almost within the distillation range of diesel fuel, whereas flash point is only 25 °C. DBE at 5 vol-% concentration resulted in a 5% reduction in PM emission with direct-injection car. 2-ethoxy ethanol has been found as an effective diesel fuel extender at 2.5, 5 and 7.5 wt-% blends in terms of exhaust emissions. Boiling point of 2-ethoxy ethanol is 135 °C. (Arteconi et al. 2011, Beeckmann et al. 2010, Subramanian et al. 2009, Nord 2005, Cheng et al. 2002, Delfort et al. 2002, Bailey et al. 1997).
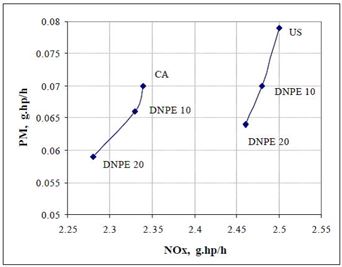
Mono-ethers give a good compromise between cetane characteristics and the behavior at low temperature. Di-n-pentyl ether (DNPE) has been reported to be a potential oxygenate for blending with diesel fuel. Cetane number of DNPE is high, 103 - 153, and other properties are diesel-like. DNPE is fully soluble in diesel fuel, whereas solubility in water is low. DNPE is 15 times more biodegradable than MTBE. DNPE could be produced, for example, from 1-pentanol, n-butane, or from methane via propylene oxide pathway. Favorable engine performance for DNPE has been reported. For the heavy-duty engine, PM, CO and HC emissions reduced by 15 - 20%, and NOx emission by 2 - 2.5% with fuel containinng 10-20% of DNPE (Figure 4). In the car tests, DNPE reduced PM emissions by nearly 10% without compromising other emissions, also under cold conditions. DNPE resembles high cetane paraffinic hydrocarbons as concerns engine performance and exhaust emissions. (Marchionna et al. 2000, Tejero et al. 2006, Murphy 1999, 2002, Martin et al. 1997, Giavazzi 1991, Pecci et al. 1991).
Figure 4. Trade-off between PM and NOx emissions by using DNPE as diesel fuel component for the heavy-duty engine. (Marchionna 2000)
Ryu et al. (2000) finds poly-ethers with high molecular weight as better diesel fuel components than mono-ethers. Dibutoxymethane (butylal) is soluble with diesel fuel. Its boiling point is 180 °C and flash point 62 °C. Cetane number of butylal is high (>74), but lubricity additive is needed. Diesel-butylal mixtures reduced engine exhaust opacity without increasing NOx emission when compared with diesel fuel. (Murphy 1999, 2002, Bertola et al. 2000).
Di-n-pentoxy methane (DNPM) has been found as favorable diesel fuel component reducing PM emission at a 25% blend in the tests with a passenger car. Boiling point of DNPM is 218 °C and cetane number is 97. (Giavazzi 1991).
Ten acetals and polyacetals at 1 - 2 wt-% oxygen contents were studied with a direct-injection car. The most significant PM reductions, 8-15%, were achieved with blends containing 5 vol-% of di-ethoxy-1,1-pentane, di-ethoxy-1,1-butane, di-ethoxy-1,1-propane or di(ethoxy-2-ethyl)polyoxomethylene. Diethoxy butane, which could be produced from ethanol and butadiene, has a high cetane number of 97, but a flash point of only 45 °C. (Delfort et al. 2002, McCormick et al. 2002)
Many other ethers have also been studied as diesel oxygenates. For example, 2-ethoxy ethyl ether (diethyl carbitol), trioxane (cyclic ether), and trimethoxy methane (McCormick et al. 2002, Murphy 1999, Yeh et al. 2001). Snamprogetti’s “oxy-diesel”, a poly-oxy-methylene, is achieved via oligomerization of dimethoxy methane (DMM) It is claimed to resemble diesel fuel and to be more biodegradable than MTBE. (Hart Diesel Fuel News 2001). MAN has tested oxymethylene ethers (OME) as diesel fuel additives. These are relatively easy to synthesize from methanol. (Lumpp et al. 2011). Boot et al. (2009) found good combustion behaviour for cyclohexanone.
Glycol ethers
Glycol ethers are produced from ethylene oxide (E-series) or from propylene oxide (P-series). (Glycol ethers online 2011).
For diglyme, good engine performance, low smoke and low NOx emissions are reported. 14.4 vol-% diglyme blend resulted in a 20 - 40% reduction in PM emission with a light-duty truck. In addition, HC and CO emission reduced without increase in NOx, benzene, butadiene, formaldehyde and PAH emissions (Ren et al. 2007, Loganathan et al. 2007, Zhu et al. 2003). A mixture of 20/80 monoglyme and diglyme (CETANERTM, cetane number >100) at 15% concentration reduced PM by 42% and NOx by 8.8% with a direct-injection diesel engine (Hess et al. 2000). Glyme, diglyme and triglyme at 5 vol-% blends (around 2 wt-% oxygen) reduced PM, CO, and HC emissions, whereas NOx emission increased by 2 - 8% with a Euro 4 passenger car. Diglyme was the best oxygenate offering 13% PM reduction, while NOx increased only 2.5%. In another study, triglyme and tetraglyme reduced PM emission by 5 - 6.5%, and tetraglyme decreased NOx emission by 2.5%. 1 wt-% oxygen obtained with glymes resulted in 6 - 7% reduction in PM emission. (Kozak et al. 2007, Natarajan et al. 2001).
Nabi and Hustad (2010) studied diethylene diglyme and jatropha biodiesel at oxygen level of 2.3 wt-% with a Euro II engine. A PM reduction was close to 30% with both fuels. CO and HC emissions were reduced as well, but NOx increased slightly or remained unchanged.
Liotta et al. (1993) found glycol ethers more effective than alcohols in reducing PM emission at the same fuel oxygen level, however, at cost of increased NOx emissions. In the opposite, Yeh et al. (2001) found lower PM emission for isodecanol than for esters, carbonates, alcohols, and ethers at 2 wt-% oxygen level.
Tripropylene glycol monoethyl ether (TPGME), dibutyl maleate (DBM) and carbonates
Natarajan et al. (2001) reviewed 71 oxygenates, of which 8 oxygenates were selected for engine tests. The criteria was to achieve 7 wt-% oxygen content in blend by using in maximum 20 vol-% of oxygenate. Therefore oxygenates with oxygen content below 35 wt-% were excluded. From the selected eight oxygenates, tripropylene glycol monomethyl ether (TPGME) and di-butyl maleate (DBM) were deemed to be the most promising ones. The other selected oxygenates were methoxy-2-propanol, dipropylene glycol monomethyl ether, 2-ethoxy ethyl acetate, 2-ethoxy ethyl ether, di-ethyl adipate and tributyrin. TPGME and DBM are miscible in aromatic diesel fuel, but the former only up to 30 vol-% and the latter only up 10 vol-% in paraffinic diesel fuel. Addition of water may lead to phase separation. (Murphy 2002, Gonzáles et al. 2001).

DBM and diethyl maleate reduced in-cylinder soot by 12%, and NOx by 5 - 8.5% in the tests with a single-cylinder engine, but not with a direct-injection light-duty engine (Natarajan et al. 2001). Stoner and Litzinger (1999) found maleates more effective than glycol ethers in soot and NOx reduction. This was thought to be due to delayed start of combustion. In the opposite, Mueller et al. (2003) found TPGME more effective than DBM in soot reduction with a single-cylinder engine. DBM produced ethylene, a soot precursor, in combustion.
With a passenger car, the best PM/NOx trade-off results were achieved diethyl maleate, DMC and DEC from a set of 7 glycols, 2 maleates and 2 carbonates at 5 vol-% concentrations. DEC was the most effective oxygenate in reducing PM emission, whereas CO and HC emissions increased. (Kozak et al. 2008, 2009). Delfort et al. (2002) found carbonates more efficient in reducing PM emissions than ethers and acetals (tetra-hydrofurfuryl derivatives) with a direct-injection diesel car. DEC, dipentyl carbonate and two ether carbonates resulted in 12-17% reduction in PM emission at 5 vol-% blends, and almost 30% reduction at 10 vol-% blends. Also other tests have shown reduced PM emissions for DMC blends, whereas contradictory NOx results have been achieved. (Keyu et al. 2002, Huang et al. 2003). Subramanian et al. (2009) found 2-ethoxy ethanol more efficient than DEC or diethyl ether at 2.5, 5, and 7.5 wt-% concentrations in terms of exhaust emissions.
Guo et al. (2005) synthesized methyl 2-ethoxyethyl carbonate (MEEC) by introducing an ether group to dimethyl carbonate. Smoke, NOx and CO emissions reduced substantially when 15-25 vol-% MEEC was added into diesel fuel in the tests with a single-cylinder engine. Viscosity of fuel decreased with addition of MEEC.
Aakko-Saksa, P., Brink, A., Happonen, M., Heikkilä, J., Hulkkonen, T., Imperato, M., Kaario, O., Koponen, P., Larmi, M., Lehto, K., Murtonen, T., Sarjovaara, T., Tilli, A., Väisänen, E., Future Combustion Technology for Synthetic and Renewable Fuels in Compression Ignition Engines (REFUEL) - Final report. 2012. Aalto University publication series: 21/2012. http://urn.fi/URN:ISBN:978-952-60-4942-7
Arteconi, A., Mazzarini, A. and Di Nicola, G. (2011) Emissions from ethers and organic car-bonate fuel additives: A review. Water Air Soil Pollution. Published online 9 April 2011.
Bailey, B., Eberhardt, J., Goguen, S. and Erwin, J. Diethyl ether (DEE) as a renewable diesel fuel. Society of Automotive Engineers. Technical Paper 972978.
Beeckmann, J., Aye, M. and Peters, N. (2010) Experimental investigation of the spray characteristics of di-n-butyl ether (DNBE) as an oxygenated compound in diesel fuel. Society of Automotive Engineers. Technical Paper 2010-01-1502.
Bertola, A. and Boulouchos, K. Oxygenated fuels for particulate emission reduction in heavy-duty DI diesel engines with common-rail fuel injection. Society of Automotive Engineers. Technical paper 2000-01-2885.
Bertoli, C., Del Giacomo, N. and Beatrice, C. (1997) Diesel Combustion Improvements by the Use of Oxyggenated Synthetic Fuels. Society of Automotive Engineers. Technical Paper 972972.
Biofine Inc. information.
BIOSCOPES (2007) Bioethanol use in the diesel sector. Rehnlund, B. Egebäck, K-E., Rydén, C., Ahlvik, p. Heavy-duty ethanol engines (Lot 2). http://ec.europa.eu/energy/renewables/biofuels/doc/standard/lot2.pdf Hamelinck, C., Schober, S., Mittelbach, M., Verolet, J., Dehue, B. Fatty acid ethyl esters. (Lot 3a) http://ec.europa.eu/energy/renewables/biofuels/doc/standard/lot3a.pdf
Chacartegui, C., Lopez, J., Alfonso, F., Aakko, P., Hamelinck, C., van der Vossen, G. and Kattenwinkel, H. Blending ethanol in diesel. (Lot 3b) http://ec.europa.eu/energy/renewables/biofuels/doc/standard/lot3b.pdf
Boot, M., Frijters, P., Klein-Douwel, R. and Baert, R. (2007) Oxygenated fuel composition impact on heavy-duty diesel engine emissions. Society of Automotive Engineers. Technical Paper 2007-01-2018.
Boot, M., Frijters, P., Luijten, C., Somers, B., Baert, R., Donkerbroek, A., Klein-Douwel, R. and Dam, N. (2009) Cyclic Oxygenates: A new class of second-generation biofuels for diesel engines? Energy & Fuels 2009, 23, 1808-1817.
Bowman, C. et al. (2006) Optimisation of Synthetic Oxygenated fuels for Diesel Engines. In GCEP Technical Report 2006.
Chang, D. and Gerpen, J. (1997) Fuel Properties and Engine Performance for Biodiesel Pre-pared from Modified Feedstocks. Society of Automotive Engineers, Warrendale. SAE Paper 971684.
Cheng, A., Dibble, R. and Buchholz, B. The effect of oxygenates on diesel engine particulate matter. SAE Technical Paper 2002-01-1705.
Delfort, B., Durand, I., Jaecker-Voirol, A., Lacôme, T., Paillé, F. & Montagne, X. (2002) Oxygen-ated compounds and diesel engine pollutant emissions performances of new generation products. Society of Automotive Engineers, Warrendale. SAE Technical Paper 2002-01-2852.
Di Serio, M., Casale, L., Tesser, R. and Santacesaria, E. (2010) New process for the production of glycerol tert-butyl ethers. Energy fuels 2010, 24, 4668--4672.
Frijters, P. and Baert, R. (2006) Oxygenated fuels for heavy-duty diesel engines. Int. J. Vehicle Design, Vol. 41, Nos 1/2/3/4. 2006.
García, E., Laca, M., Pérez, E., Garrido, A. and Peinado, J. (2008) New class of acetal derived from glycerin as a biodiesel fuel component. Energy & Fuels 2008, 22, 4174-4280.
Giavazzi, F., Patrini, R., Ancillotti, F., Pecci, G.C., Treré, R. and Benelli, M. (1991). Oxygenated diesel fuels. Part 2 – Practical aspects of their use. Proceedings of the Ninth International Symposium on Alcohol Fuels (ISAF), Firenze, 1991. p. 327-335.
Glycol ethers online (2010). http://www.glycol-ethers.eu/what-are-glycol-ethers/glycols-ethers-in-everyday-life.
Golubkov, I. et al. (2005) Alcohol-based diesel fuel for conventional engines – it is a reality. ISAF XV. The International Symposia on Alcohol Fuels and Other Renewables. San Diego.
Gonzáles, M. et al. (2001) Oxygenates screening for Advanced Petroleum-Based Diesel Fuels: part 2. The effect of Oxygenate Blending Compounds on Exhaust Emissions. Society of Automotive Engineers. Technical Paper 2001-01-3632.
Guo, H., Huang, X., Zhang, J., Zhang, H. and li, L. Study of methyl 2-ethoxyethyl carbonate used as an oxygenated diesel fuel. Society of Automotive Engineers. Technical Paper 2005-01-3142.
Hart Diesel Fuel News 2001, July 2001.
Hashimoto, T. and Akasaka, Y. (1991) Evaluation of oxygenated fuels using conventional and a new type of diesel engines. Proceedings of 9th ISAF, Firenze. 336-341.
Hess, H.S., Szybist, J., Boehman, A.L., Tijm, P.J.A. and Waller, F.J. (2000). The Use of CETANERTM for the Reduction of Particulate Matter Emissions in a Turbocharged Di-rect Injection Medium-Duty Diesel Engine. Seventeeth Annual
International Pittsburgh Coal Conference. Pittsburgh, PA, September 11-15, 2000.
Hilden, D., Eckstrom, J. and Wolf, L. (2001) The emissions performance of oxygenated diesel fuels in a prototype DI diesel engine. SAE Technical Paper 2001-01-0650.
Huang, Z., Jiang, D., Zeng, K., Liu, B. and Yang, Z. (2003) Combustion characteristics and heat release analysis of a direct injection compression ignition engine fuelled with diesel-dimethyl carbonate blends. Proceedings of the Institution of Mechanical Engineers, Part D: Journal of Automotive Engineering 2003 217:595.
Hu, C., Jian-Xin, W., Shi-Jin, S., Xin-Liang, A. and Wen-Miao, C. (2006) Effects of Ethanol in Ester- Ethanol -Diesel Blended Fuels on Spray Behavior and PM emission. SAE Tech-nical Paper 2006-01-0236.
Janssen, A., Muether, M., Pischinger, S., Kolbeck, A. and Lamping, M. (2009) Tailor-made fuels: the potential of oxygen content in fuels for advanced combustion systems. Society of Automotive Engineers. Technical Paper 2009-01-2765.
Karas, L., Kesling, H., Liotta, F. and Nandi, M. (1994) Low emission oxygenated diesel fuel. San Diego.
Keyu, P., Cheung, C.S., Liu Ming An, Lee, S.C. (2002). Performance of DMC blended diesel in reducing pollutant emissions. Better Air Quality in Asian and Pacific Rim Cities, 16 – 18 December 2002.
Kozak, M., Merkisz, J., Bielaczyc, P. and Szczotka, A. (2009) The influence of oxygenated diesel fuels on a diesel vehicle PM/NOx emission trade-off. Society of Automotive Engineers, Technical Paper 2009-01-2696.
Kozak, M. and Merkisz, J. (2008) The influence of synthetic oxygenates on Euro IV diesel passenger car exhaust emissions. Society of Automotive Engineers, Warrendale. SAE Technical Paper 2008-01-1813 (Part 2) and 2008-01-2387 (Part 3).
Kozak, M., Merkisz, J., Bielaczyc, P. and Szczotka, A. (2007) The influence of synthetic oxygenates on Euro IV diesel passenger car exhaust emissions. Society of Automotive Engineers, Warrendale. SAE Technical Paper 2007-01-0069.
Lin, C. Y. and Huang, J. C. (2003). An oxygenating additive for improving the performance and emission characteristics of marine diesel engines. Ocean Engineering. Volume 30, Issue 13 , September 2003, Pages 1699-1715.
Liotta, F. and Montalvo, D. The effect of oxygenated fuels on emissions from a modern heavy-duty diesel engine. Society of Automotive Engineers. Technical Paper 932734.
Loganathan, S., Tamilporai, P., Vijayan, K. and Sujithradevi, B. (2007) Simulation and analysis of effect of oxygenate blended diesel on combustion and performance in turbocharged diesel engine. Society of Automotive Engineers, Warrendale. SAE Technical Paper 2007-01-2019.
Lumpp, B., Rothe, D., Pastötter; C., Lämmermann, R. and Eberhard J. (2011) Oxymethylene Ethers as Diesel Fuel Additives of the Future. MTZ worldwide Edition, 2011-03.
Marchionna, M. and Patrini, R. (2000) High cetane ethers for the reformulation of diesel fuels. 16th World Petroleum Congress.
Marchionna, M., Patrini, P., Sanfilippo, D., Paggini, A., Giavazzi, F. and Pellegrini, L. (2001) From natural gas to oxygenates for cleaner diesel fuels. Studies in Surface Science and Catalysis. Volume 136, 2001, Pages 489-494.
Martin, B., Aakko, P., Beckman, D., del Giacomo, N. and Giavazzi, F. (1997) Influence of future fuel formulations on diesel engine emissions - a joint European study. SAE SAE Technical Paper 972966.
McCormick, R., et al. (2002) Performance and Properties of Renewable Diesel Fuels. In FY 2002 Progress Report of “Fuels for Advanced CIDI Engines. November 2002.
McCormic, R. and Parish, R. (2001) Technical barriers to the use of ethanol in diesel fuel. National Renewable Energy Laboratory. Colorado 2001. (Report NREL/MP 540-32674).
McEnally, C. and Pfefferle, L. (2011) Sooting Tendencies of Oxygenated Hydrocarbons in Laboratory-Scale Flames. Environ. Sci. Technol. 2011, 45, 2498-2503.
Mohammadi, A., Kee, S-S., Ishiyama, T., Kakuta, T. and Matsumoto, T. (2005) Implementation of Ethanol Diesel Blend Fuels in PCCI Combustion. SAE Technical Paper 2005-01-3712.
Mohammadi, A., Ishiyama, T., Kawanabe, H. and Horibe, N. (2004) An Optimal Usage of Recent Combustion Control Technologies for DI Diesel Engine Operating on Ethanol Blended Fuels. SAE Technical Paper 2001-01-1866. 5
Mueller, C.J. et al. (2003) Effects of Oxygenates on Soot Processes in DI Diesel Engines : Experiments and Numerical Simulations. SAE 2003-01-1791.
Murphy, M. (1999) Safety and industrial hygiene issues related to the use of oxygenates in diesel fuel. SAE Technical Paper 1999-01-1473.
Murphy, M. (2002) Oxygenate compatibility with diesel fuels. Society of Automotive Engineers. Technical Paper 2002-01-2848.
Nabi, N and Hustad, J. (2010) Effect of fuel oxygen on engine performance and exhaust emis-sions including ultrafine particle fueling with diesel-oxygenate blends. Society of Au-tomotive Engineers. Technical Paper 2010-01-2130.
Natarajan, M., Frame, E., Naegeli, D., Asmus, T., Clark, W., Garbak, J., Gonzáles, M., Liney, E., Piel, W. and Wallace, J. (2001) Oxygenates for Advanced Petroleum-Based Diesel Fuels: Part 1. Screening and Selection Methodology for the Oxygenates. Society of Automotive Engineers, Warrendale. SAE Technical Paper 2001-01-3631.
Nord, K. (2005) Particles and unregulated emissions from CI engines subjected to emission control. Luleå University of Tecnology. Doctoral Thesis. 2005:09.
Nylund, N.-O., Aakko, P., Niemi, S., Paanu, T. and Berg, R. (2005) Alcohols/ethers as oxygenates in diesel fuel: Properties of blended fuels and evaluation of practical experiences. IEA Advanced Motor Fuels, Task 26. Report TEC 3/2005.download report
Pecci, G., GiClerici, M., Giavazzi, F., Ancillotti, F., Marchionna, M. and Patrini, R. (1991). Oxy-genated diesel fuels. Part 1 – Structure and properties correlation. Proceedings of the Ninth International Symposium on Alcohol Fuels (ISAF), Firenze, 1991. p. 321-6.
Petterson, O. (2005) Fleet test of Agrodiesel 15, a partly renewable diesel fuel. JTI – Swedish Institute of Agricultural and Environmental Engineering.
Ren, Y., Huang, Z., Miao, H., Jiang, D., Zeng, K., Liu, B. and Wang, X. (2007) Effect of the addition of diglyme in diesel fuel on combustion and emissions in a compression-ignition engine. Energy & Fuels 2007, 21, 2573 2583.
Sebastian, J. and Nagarajan, G. (2010) Experimental Study on Influence of Fuel Oxygen Content on Combustion and Emission Characteristics of a Direct Injection C.I. Engine. SAE Technical Paper 2010- 01-1969
Serdari, A., Lois, E. and Stournas, S. Tertiary fatty amides as diesel fuel substitutes. Int. J. Energy Res. 2000: 24: 455-466.
Shell Lexikon - Verbrennungsmotor. Ein Supplement von ATZ und MTZ - Folge 26.Souligny, M., Graham, L. and Rideout, G. (2004). Heavy-Duty Diesel Engine Performance and Comparative Emission Measurements for Different Biodiesel Blends Used in the BIOBUS Project. SAE Technical Paper 2004-01-1861.
Shudo, T., Fujibe, A., Kazahaya, M., Aoyagi, Y., Ishii, H., Goto, Y. and Noda, A. (2005) The Cold Flow Performance and the Combustion Characteristics with Ethanol Blended Bi-odiesel Fuel. SAE Technical Paper 2005-01-3737.
Smith, B., Ott, L. and Bruno, T. (2008) Composition-explicit distillation curves of diesel fuel with glycol ether and glycol ester oxygenates: fuel analysis metrology to enable decreased particulate emissions. Environ. Sci. Technol. 2008, 42, 7682 - 7689.
Spooner-Wyman, J., Appleby, D. and Yost, D. (2003) Evaluation of di-butoxy glycerol (DBG) for use as a diesel fuel blend component. SAE Technical Paper 2003-01-2281.
Stoner, M. and Litzinger, T. Effects of Structure and Boiling Point of Oxygenated Blending Compounds in Reducing Diesel Emissions. SAE Technical Paper 1999-01-1475.
Stournas, S., Lois, E., Serdari, A. & Gouli, S. (1993). Novel oxygenates in gasoline and diesel fuel for improved ignition quality and reduced emissions. Laboratory of Fuel and Lubricant Technology. National Technical University, Athens, Greece. ISAF 1993.
Subramanian, M., Chandrasekaran, S. and Rajesh, M. The effect of oxygenated diesel blends on combustion process and performance parameters in a single cylinder diesel engine. SAE Technical Paper 2009-01-1681.
Tejero, J. et al. (2006) Dehydration of 1-pentanol to di-n-pentyl ether catalysed by a mi-croporous ion-exchange resin with simultaneous water removal. Applied Catalysis A: General 308 (2006) 223-230.
Valentino, G., Corcione, F., Ianuzzi, S. and Serra, S. (2012) Experimental study on performance and emissions of a high speed diesel engine fuelled with n-butanol diesel blends under premixed low temperature combustion. Fuel 92 (2012) 297-307.
Wadumesthrige, K., Ng, S. and Salley, S. (2010) Properties of butanol-biodiesel-ULSD ternary mixtures. Society of Automotive Engineers. SAE 2010-01-2133.
Xu, Y. and Lee, C. (2006) Study of soot formation of oxygenated diesel fuels using forward illumination light extinction (FILE) technique. Society of Automotive Engineers. Technical Paper 2006-01-1415.
Yanfeng, G., Shenghua, L., Hejun, G., Tiegang, H. and Longbao, Z. (2006) A new diesel oxy-genate additive and its effects on engine combustion and emissions. Applied Thermal Engineering 27 (2007) 202-207.
Yeh, L., Rickeard, D., Duff, J., Bateman, J., Schlosberg, R. and Caers, R. Oxygenates: An evaluation of their effects on diesel emissions. SAE Technical Paper 2001-01-2019.
Zhu, J., Cao, X-L., Pigeon, R. and Mitchell, K. (2003). Comparison of Vehicle Exhaust Emissions from Modified Diesel Fuels. Journal of the Air and Waste Management Association. Volume 53. January 2003.
Zöldy, M., Hollo, A. and Thernesz, A. (2010) Butanol as diesel extender option for internal com-bustion engines. SAE Automotive Engineers. Technical Paper 2010-01-0481.